
Chemistry, 17.05.2021 09:40 xxtonixwilsonxx
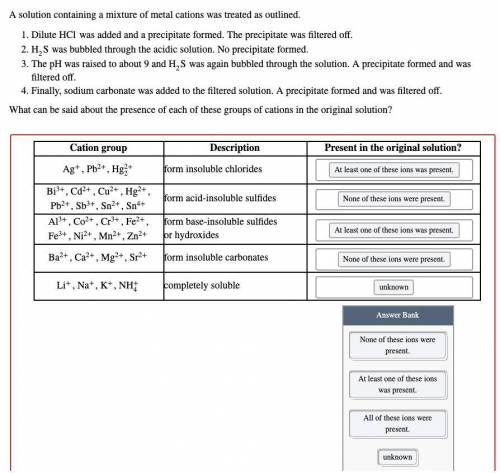
A solution containing a mixture of metal cations was treated as outlined.
Dilute HCl was added and a precipitate formed. The precipitate was filtered off.
H2S was bubbled through the acidic solution. No precipitate formed.
The pH was raised to about 9 and H2S was again bubbled through the solution. A precipitate formed and was filtered off.
Finally, sodium carbonate was added to the filtered solution. A precipitate formed and was filtered off.
What can be said about the presence of each of these groups of cations in the original solution?
Options: None of these ions were present
At least one of these ions was present
All of these ions were present
Unknown

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3


Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
A solution containing a mixture of metal cations was treated as outlined.
Dilute HCl was added and...
Questions



Mathematics, 03.11.2019 19:31

English, 03.11.2019 19:31


Arts, 03.11.2019 19:31

Mathematics, 03.11.2019 19:31





English, 03.11.2019 19:31


Mathematics, 03.11.2019 19:31


Chemistry, 03.11.2019 19:31


Computers and Technology, 03.11.2019 19:31

Mathematics, 03.11.2019 19:31



