
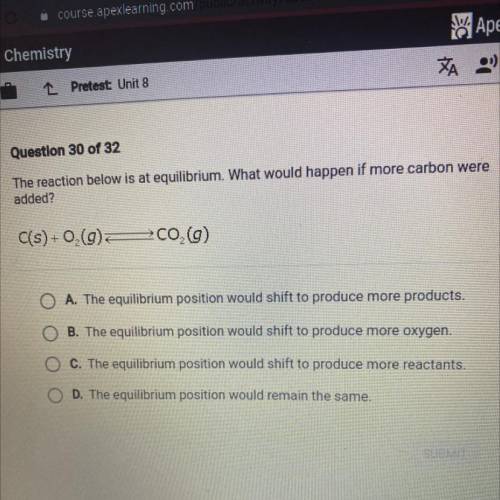
The reaction below is at equilibrium. What would happen if more carbon were
added?
C(s) +0,(9)
700 (9)
A. The equilibrium position would shift to produce more products.
B. The equilibrium position would shift to produce more oxygen.
C. The equilibrium position would shift to produce more reactants.
D. The equilibrium position would remain the same.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1
You know the right answer?
The reaction below is at equilibrium. What would happen if more carbon were
added?
C(s) +0,(9...
C(s) +0,(9...
Questions



English, 26.08.2020 02:01

History, 26.08.2020 02:01



History, 26.08.2020 02:01


Mathematics, 26.08.2020 02:01

English, 26.08.2020 02:01


History, 26.08.2020 02:01




History, 26.08.2020 02:01

English, 26.08.2020 02:01

English, 26.08.2020 02:01




