On 2
6 pts
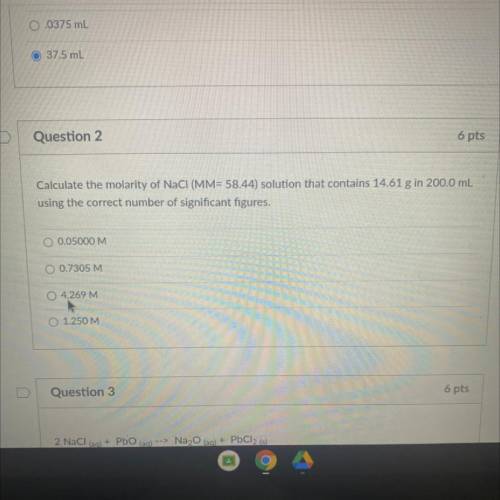
Calculate the molarity of NaCl (MM= 58.44) solution that contains 14.61 g in 200,0...

Chemistry, 17.05.2021 22:00 xeskimopie
On 2
6 pts
Calculate the molarity of NaCl (MM= 58.44) solution that contains 14.61 g in 200,0 mL
using the correct number of significant figures,
O 0.05000 M
O 0.7305 M
O 4.269 M
O 1.250 M

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
Questions




History, 24.06.2019 19:50












English, 24.06.2019 19:50

Mathematics, 24.06.2019 19:50

Mathematics, 24.06.2019 19:50


Mathematics, 24.06.2019 19:50



