
Chemistry, 17.05.2021 22:00 jumeljean123oythxy
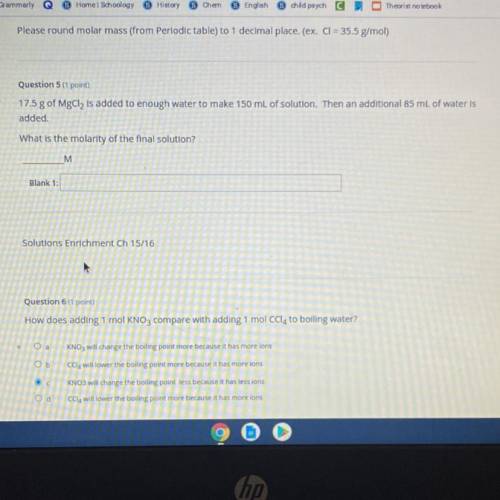
17.5 g of MgCl2 is added to enough water to make 150 mL of solution. Then an additional 85 mL of water is
added.
What is the molarity of the final solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3
You know the right answer?
17.5 g of MgCl2 is added to enough water to make 150 mL of solution. Then an additional 85 mL of wat...
Questions














Mathematics, 19.01.2021 02:00





Mathematics, 19.01.2021 02:00



