
Chemistry, 17.05.2021 22:30 itsyagirl11076
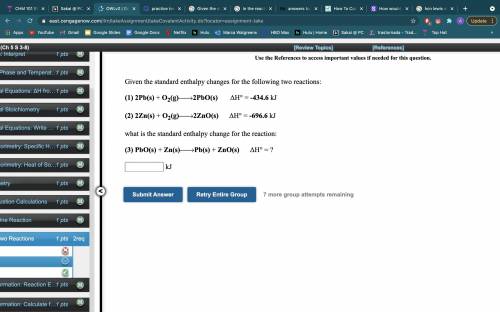
Given the standard enthalpy changes for the following two reactions:
(1) 2Pb(s) + O2(g)2PbO(s). ΔH° = -434.6 kJ
(2) 2Zn(s) + O2(g)2ZnO(s).ΔH° = -696.6 kJ
what is the standard enthalpy change for the reaction:
(3) PbO(s) + Zn(s)Pb(s) + ZnO(s).ΔH° = __kJ

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1


Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1
You know the right answer?
Given the standard enthalpy changes for the following two reactions:
(1) 2Pb(s) + O2(g)2PbO(s). ΔH°...
Questions

History, 21.09.2019 16:10



History, 21.09.2019 16:10





Physics, 21.09.2019 16:10



Biology, 21.09.2019 16:10


Mathematics, 21.09.2019 16:10

History, 21.09.2019 16:10

Mathematics, 21.09.2019 16:10






