
Chemistry, 17.05.2021 23:10 pablohc200021
0 0
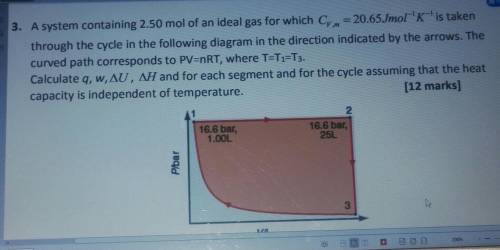
3. A system containing 2.50 mol of an ideal gas for which Cy. m = 20.65Jmol-'K-'is taken
through the cycle in the following diagram in the direction indicated by the arrows. The
curved path corresponds to PV=nRT, where T=T1=T3.
Calculate q, w, AU, AH and for each segment and for the cycle assuming that the heat
capacity is independent of temperature.
(12 marks]
2.
16.6 bar,
1.00L
16.6 bar,
25L
Plbar
یا
3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3
You know the right answer?
0 0
3. A system containing 2.50 mol of an ideal gas for which Cy. m = 20.65Jmol-'K-'is taken
t...
t...
Questions

Physics, 11.11.2020 18:10


Mathematics, 11.11.2020 18:10






Mathematics, 11.11.2020 18:10

History, 11.11.2020 18:10

History, 11.11.2020 18:10

Mathematics, 11.11.2020 18:10


Mathematics, 11.11.2020 18:10



Computers and Technology, 11.11.2020 18:10





