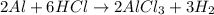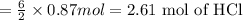Chemistry, 18.05.2021 19:20 raymondleggett44
Aluminum reacts with hydrochloric acid to produce aluminum chloride and hydrogen. Write a balanced equation for the reaction and calculate the number of moles of hydrochloric acid required to react with 0.87 mole of aluminum.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

You know the right answer?
Aluminum reacts with hydrochloric acid to produce aluminum chloride and hydrogen. Write a balanced e...
Questions




Social Studies, 12.02.2022 19:20





Mathematics, 12.02.2022 19:20

English, 12.02.2022 19:20

Social Studies, 12.02.2022 19:20






Mathematics, 12.02.2022 19:20