
Chemistry, 18.05.2021 19:20 marilynlaraaa
In completing a Fischer Esterification experiment using acetic acid and unknown alcohol catalyzed by sulfuric acid a student starts with excess acetic acid with the intention of shifting the equilibrium towards formation of product. Identify the law used to predict this effect.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1
You know the right answer?
In completing a Fischer Esterification experiment using acetic acid and unknown alcohol catalyzed by...
Questions

Mathematics, 13.04.2021 21:50

Mathematics, 13.04.2021 21:50



Mathematics, 13.04.2021 21:50

Computers and Technology, 13.04.2021 21:50


English, 13.04.2021 21:50

English, 13.04.2021 21:50


Spanish, 13.04.2021 21:50

Mathematics, 13.04.2021 21:50




Mathematics, 13.04.2021 21:50


Mathematics, 13.04.2021 21:50

English, 13.04.2021 21:50

Health, 13.04.2021 21:50

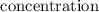 of any of the reactants or the products in the reaction at an equilibrium state, is changed, then it changes the composition of equilibrium mixture in order to minimize the effect of
of any of the reactants or the products in the reaction at an equilibrium state, is changed, then it changes the composition of equilibrium mixture in order to minimize the effect of 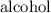 ⇄
⇄ 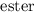 + water
+ water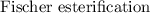 is a reaction which involves the equilibrium. So to force the reaction towards the side of
is a reaction which involves the equilibrium. So to force the reaction towards the side of 

