
Chemistry, 18.05.2021 20:30 Nismo3501037
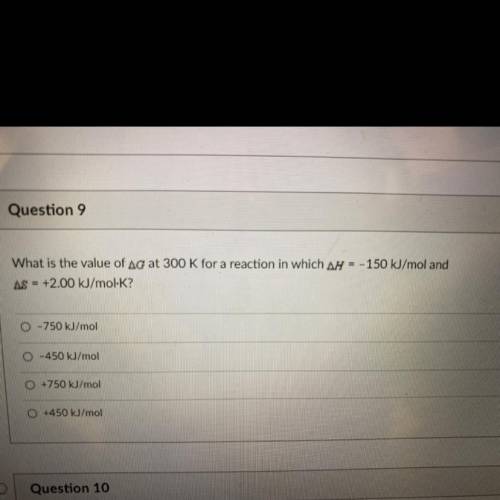
What is the value of Ag at 300 K for a reaction in which ar = - 150 kJ/mol and AS = +2.00 kJ/mol-K?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

You know the right answer?
What is the value of Ag at 300 K for a reaction in which ar = - 150 kJ/mol and
AS = +2.00 kJ/mol-K?...
Questions


Mathematics, 18.11.2020 19:40

Mathematics, 18.11.2020 19:40


Mathematics, 18.11.2020 19:40



Mathematics, 18.11.2020 19:40

Geography, 18.11.2020 19:40


Mathematics, 18.11.2020 19:40

Computers and Technology, 18.11.2020 19:40



Mathematics, 18.11.2020 19:40

Mathematics, 18.11.2020 19:40

Mathematics, 18.11.2020 19:40


History, 18.11.2020 19:40

Mathematics, 18.11.2020 19:40



