

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
You know the right answer?
Consider the following reactants:
BeCl2(aq) + 2AgNO3(aq) →
A. Predict the products and write...
A. Predict the products and write...
Questions










Mathematics, 17.12.2019 08:31

Biology, 17.12.2019 08:31




Mathematics, 17.12.2019 08:31

Mathematics, 17.12.2019 08:31

English, 17.12.2019 08:31

Mathematics, 17.12.2019 08:31


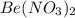 and AgCl. The balanced equation is
and AgCl. The balanced equation is 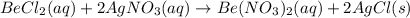 .
. = 2
= 2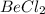 and
and 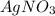 . The ions are
. The ions are  ,
, 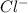 ,
, 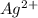 and
and  .
.

