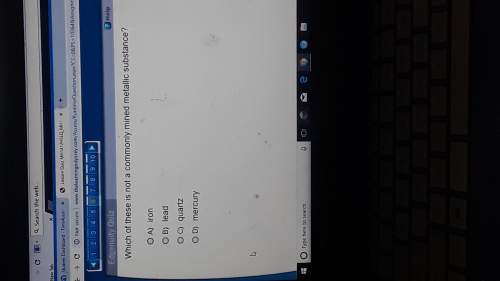
Chemistry, 18.05.2021 23:20 natasniebow
Consider the reaction 2A + 3B ⟶3C + 2D. The standard enthalpy of formation for A, B, C and D are -79.8, 49.2, -145.8, and -87.4, respectively in kJ / mol. What is the standard enthalpy change for the reaction in kJ/mol?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
This is a mixture that has the same composition throughout.
Answers: 1

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3
You know the right answer?
Consider the reaction 2A + 3B ⟶3C + 2D. The standard enthalpy of formation for A, B, C and D are -79...
Questions

Mathematics, 19.12.2019 08:31

English, 19.12.2019 08:31

Computers and Technology, 19.12.2019 08:31

Mathematics, 19.12.2019 08:31


Mathematics, 19.12.2019 08:31

Mathematics, 19.12.2019 08:31


Computers and Technology, 19.12.2019 08:31

Mathematics, 19.12.2019 08:31


Biology, 19.12.2019 08:31


Biology, 19.12.2019 08:31

History, 19.12.2019 08:31

History, 19.12.2019 08:31