Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Drag each number to the correct location on the equation. each number can be used more than once, but not all numbers will be used. balance the equation with the coefficients. 2 3 4 5 kclo3 -> kcl + o2
Answers: 1

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2
You know the right answer?
3
1 point
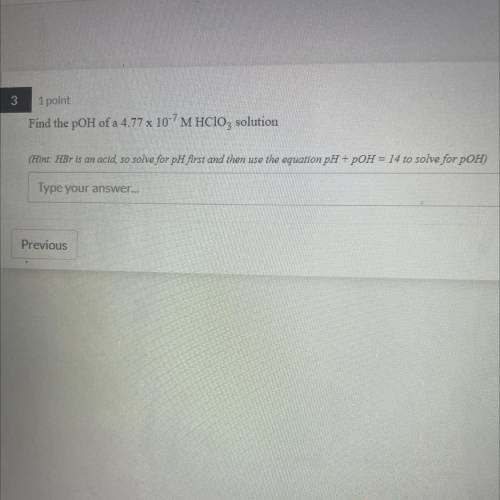
Find the pOH of a 4.77 x 10-7 M HClO3 solution
(Hint: HBr is an acid, so solv...
Find the pOH of a 4.77 x 10-7 M HClO3 solution
(Hint: HBr is an acid, so solv...
Questions

Mathematics, 02.11.2020 06:10

Mathematics, 02.11.2020 06:10

Mathematics, 02.11.2020 06:10


Geography, 02.11.2020 06:10

Mathematics, 02.11.2020 06:10




Mathematics, 02.11.2020 06:10


History, 02.11.2020 06:10

Mathematics, 02.11.2020 06:10



Biology, 02.11.2020 06:10


Mathematics, 02.11.2020 06:10

Physics, 02.11.2020 06:10