2
3 K 4
KM
5
67
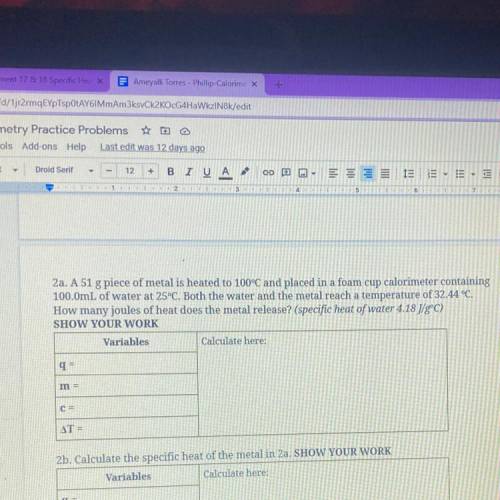
2a. A 51 g piece of metal is heated to 100°C and placed in...

Chemistry, 19.05.2021 01:00 morenoozzie
2
3 K 4
KM
5
67
2a. A 51 g piece of metal is heated to 100°C and placed in a foam cup calorimeter containing
100.0mL of water at 25°C. Both the water and the metal reach a temperature of 32.44 °C.
How many joules of heat does the metal release? (specific heat of water 4.18 J/gºC)
SHOW YOUR WORK
Variables
Calculate here:
q=
m =
C=
AT =

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1

Chemistry, 23.06.2019 09:00
Describe the process that was used in this lab to create magnesium oxide, specifically identifying the type of chemical reaction. explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
Answers: 2
You know the right answer?
Questions

Business, 01.09.2019 11:20


Spanish, 01.09.2019 11:20



Mathematics, 01.09.2019 11:20





Mathematics, 01.09.2019 11:20


English, 01.09.2019 11:20

Physics, 01.09.2019 11:20

Social Studies, 01.09.2019 11:20

Mathematics, 01.09.2019 11:20


Social Studies, 01.09.2019 11:20




