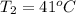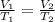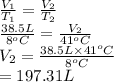An arctic weather balloon is filled with 38.5 L of helium gas inside a prep shed. The temperature inside the shed is 8. °C. The balloon is then taken outside, where the temperature is -41. °C. Calculate the new volume of the balloon. You may assume the pressure on the balloon stays constant at exactly 1 atm. Be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 04:31
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1
You know the right answer?
An arctic weather balloon is filled with 38.5 L of helium gas inside a prep shed. The temperature in...
Questions


History, 02.09.2019 00:00




History, 02.09.2019 00:00



Chemistry, 02.09.2019 00:00



Social Studies, 02.09.2019 00:00


History, 02.09.2019 00:00

History, 02.09.2019 00:00


Social Studies, 02.09.2019 00:00

Mathematics, 02.09.2019 00:00


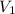 = 38.5 L,
= 38.5 L, 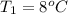
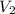 = ?,
= ?,