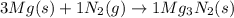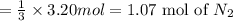Chemistry, 19.05.2021 16:00 aliami0306oyaj0n
When 3.20 moles of magnesium is heated and placed in a container of nitrogen gas the two substances undergo a synthesis reaction. How many moles of nitrogen gas will react? 3 Mg (s) + 1 N2 (g) → 1 Mg3N2 (s)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3


Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
You know the right answer?
When 3.20 moles of magnesium is heated and placed in a container of nitrogen gas the two substances...
Questions



Social Studies, 15.11.2019 15:31

Social Studies, 15.11.2019 15:31


Health, 15.11.2019 15:31



World Languages, 15.11.2019 15:31

History, 15.11.2019 15:31

Social Studies, 15.11.2019 15:31



Social Studies, 15.11.2019 15:31



History, 15.11.2019 15:31

Mathematics, 15.11.2019 15:31


History, 15.11.2019 15:31