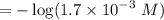Chemistry, 19.05.2021 19:10 shealwaysknows23
Calculate the concentration of H3O+ ions present in a solution of HCl that has a measured pH of 1.510 .
M
HCl, the acid in Part 1, is a strong acid. The concentration of H3O+ ions in this solution is the same as the initial concentration of the acid.
A solution of the weak acid CH3COOH with the same initial concentration of acid will have a pH that is (Higer, lower, the same) the pH of the HCl solution.
Rank the following solutions in order of increasing acidity, placing the most acidic solution at the left. (CH3COOH is approximately 1.0% ionized at this concentration.)
pH= 0.00, pH= 7.45, [HCl]= .15M, [CH3COOH]= .15M
order of acidity
most acidic > least acidic

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 23.06.2019 07:00
4. glenn andrews recently bought a motorcycle for $3,950. if he had to pay 6% sales tax on the bike, what was the total cost of the motorcycle?
Answers: 1

Chemistry, 23.06.2019 13:30
Which of these statements describes the size of an atom? a. an atom is larger than a sheet of aluminum foil. b. an atom is small but can be seen with just our eyes. c. an atom is the size of a plastic building block. d. an atom is tiny and cannot be seen without magnification.
Answers: 2
You know the right answer?
Calculate the concentration of H3O+ ions present in a solution of HCl that has a measured pH of 1.51...
Questions


Mathematics, 01.02.2021 23:20



Mathematics, 01.02.2021 23:20

Mathematics, 01.02.2021 23:20


Mathematics, 01.02.2021 23:20

Mathematics, 01.02.2021 23:20

Mathematics, 01.02.2021 23:20

Mathematics, 01.02.2021 23:20


Mathematics, 01.02.2021 23:20

Mathematics, 01.02.2021 23:20



Advanced Placement (AP), 01.02.2021 23:20

Social Studies, 01.02.2021 23:20

Biology, 01.02.2021 23:20

![$[H_3O^+]$](/tpl/images/1334/2671/dab07.png) is pH =
is pH = ![$- \log [H_3O^+]$](/tpl/images/1334/2671/b0a97.png)
![$[H_3O^+]=10^{-pH}$](/tpl/images/1334/2671/b447d.png)
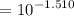
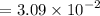
![$=- \log[0.15]$](/tpl/images/1334/2671/efddc.png)
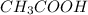 is as follows :
is as follows :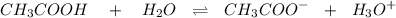
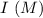 0.15 0 0
0.15 0 0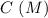 -x +x +x
-x +x +x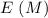 0.15 - x x x
0.15 - x x x![$K_a=\frac{[CH_3COO^-][H_3O^+]}{[CH_3COOH]}$](/tpl/images/1334/2671/7fb51.png)
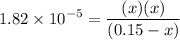
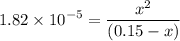
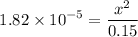
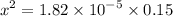
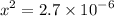
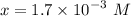
![$[H_3O^+] =x=1.7 \times 10^{-3} \ M$](/tpl/images/1334/2671/663fb.png)