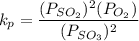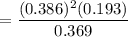Chemistry, 19.05.2021 19:10 sarahaziz9526
A student ran the following reaction in the laboratory at 1080 K: 2SO3(g) 2SO2(g) + O2(g) When she introduced SO3(g) at a pressure of 0.948 atm into a 1.00 L evacuated container, she found the equilibrium partial pressure of SO3(g) to be 0.369 atm. Calculate the equilibrium constant, Kp, she obtained for this reaction. Kp =

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3


Chemistry, 23.06.2019 06:50
What is the volume of 3.2 moles of chlorine gas (cl2) at 295 k and 1.1 atm?
Answers: 1
You know the right answer?
A student ran the following reaction in the laboratory at 1080 K: 2SO3(g) 2SO2(g) + O2(g) When she i...
Questions

Mathematics, 12.01.2021 22:20


English, 12.01.2021 22:20



Mathematics, 12.01.2021 22:20

English, 12.01.2021 22:20

Mathematics, 12.01.2021 22:20


Mathematics, 12.01.2021 22:20


Chemistry, 12.01.2021 22:20

Biology, 12.01.2021 22:20

Mathematics, 12.01.2021 22:20


Mathematics, 12.01.2021 22:20




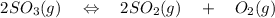
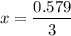
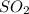 = 0.193 x 2
= 0.193 x 2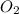 = 0.193 atm
= 0.193 atm