Redox reaction:
HNO3 +S → H2SO4 + NO
Half-reactions with balanced electrons:
6e + 2HNO3...

Chemistry, 19.05.2021 19:30 camballard3848
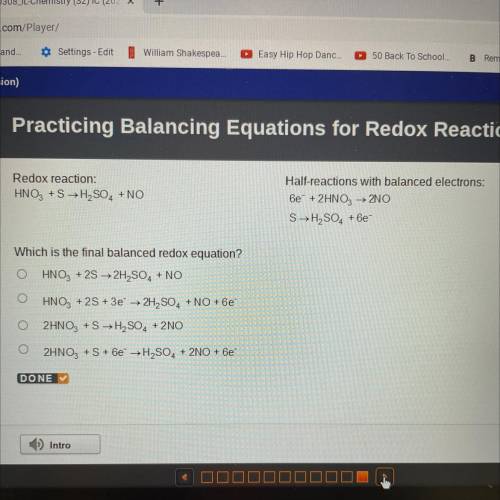
Redox reaction:
HNO3 +S → H2SO4 + NO
Half-reactions with balanced electrons:
6e + 2HNO3 → 2NO
SH2SO4 +6e
Which is the final balanced redox equation?
HNO3 + 2S → 2H2SO4 + NO
HNO3 + 2S + 3e → 2H2SO4 + NO + 6e
2HNO3 +S →H2SO4 + 2NO
2HNO3 + S + 6e → H2SO4 + 2NO + 6e

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
You know the right answer?
Questions


Mathematics, 07.12.2020 23:20

History, 07.12.2020 23:20

Mathematics, 07.12.2020 23:20

English, 07.12.2020 23:20




Mathematics, 07.12.2020 23:20



Chemistry, 07.12.2020 23:20

Medicine, 07.12.2020 23:20

History, 07.12.2020 23:20


Mathematics, 07.12.2020 23:20

Biology, 07.12.2020 23:20

Mathematics, 07.12.2020 23:20


Mathematics, 07.12.2020 23:20



