Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3


Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

You know the right answer?
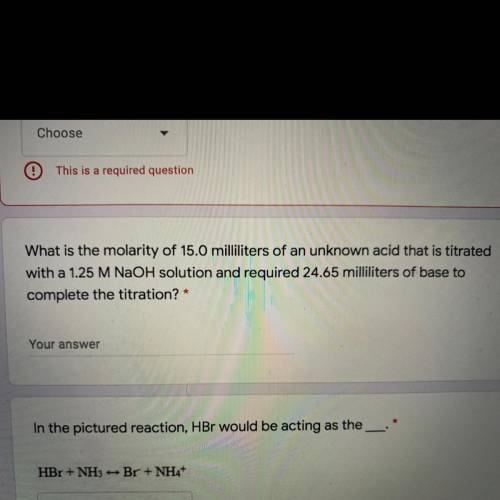
What is the molarity of 15.0 milliliters of an unknown acid that is titrated
with a 1.25 M NaOH sol...
Questions





Mathematics, 19.11.2020 20:10

Mathematics, 19.11.2020 20:10


Mathematics, 19.11.2020 20:10





Chemistry, 19.11.2020 20:10


Social Studies, 19.11.2020 20:10

Mathematics, 19.11.2020 20:10


Mathematics, 19.11.2020 20:10