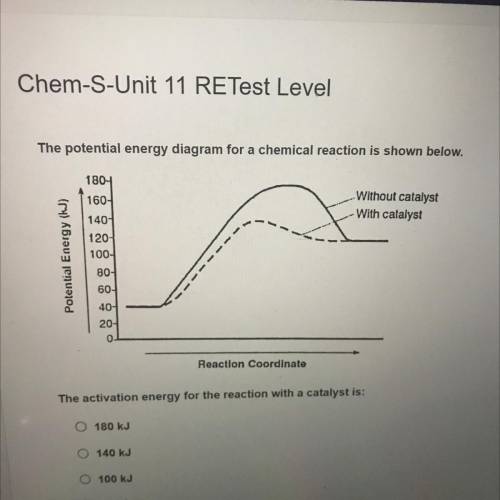
The potential energy diagram for a chemical reaction is shown below.
Without catalyst
With ca...

Chemistry, 20.05.2021 17:20 AnonymousLizard52303
The potential energy diagram for a chemical reaction is shown below.
Without catalyst
With catalyst
Potential Energy (J)
1804
160-
140
120-
100-
80-
60-
40-
20-
0
Reaction Coordinato
The activation energy for the reaction with a catalyst is:
O 180 kJ
140 kJ
100 kJ
60 kJ

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1

Chemistry, 21.06.2019 18:30
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
You know the right answer?
Questions

Mathematics, 11.05.2021 23:20

English, 11.05.2021 23:20

Mathematics, 11.05.2021 23:20

History, 11.05.2021 23:20





Mathematics, 11.05.2021 23:20


Mathematics, 11.05.2021 23:20


Mathematics, 11.05.2021 23:20



Social Studies, 11.05.2021 23:20




Arts, 11.05.2021 23:20



