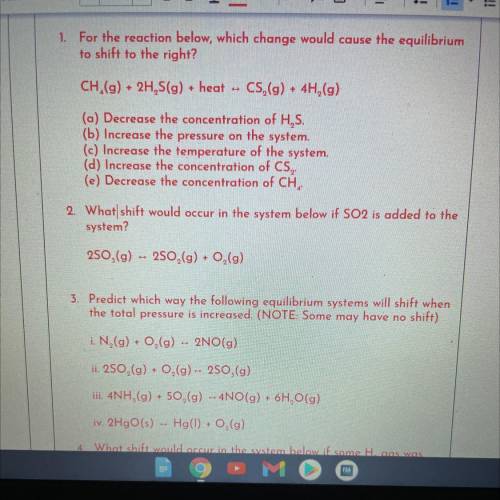
1. For the reaction below, which change would cause the equilibrium
to shift to the right?
CH...

Chemistry, 20.05.2021 19:00 okokalyssa
1. For the reaction below, which change would cause the equilibrium
to shift to the right?
CH.(g) + 2H, S(g) + heat
CS,(g) + 4H,(g)
(a) Decrease the concentration of H, S.
(b) Increase the pressure on the system.
(c) Increase the temperature of the system.
(d) Increase the concentration of CS,
(e) Decrease the concentration of CH,
can someone also help me answer the rest of the questions so i can check my work thank you

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

You know the right answer?
Questions

Mathematics, 18.09.2019 08:20


Mathematics, 18.09.2019 08:20

English, 18.09.2019 08:20

Mathematics, 18.09.2019 08:20

Physics, 18.09.2019 08:20

Biology, 18.09.2019 08:20






Chemistry, 18.09.2019 08:20



Mathematics, 18.09.2019 08:20


Social Studies, 18.09.2019 08:20


History, 18.09.2019 08:20



