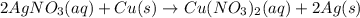Chemistry, 20.05.2021 19:20 deidaraXneji
Study the reaction.
2AgNO3 (aq)+Cu (s)→Cu(NO3)2 (aq)+2Ag (s)
If the oxidation number of copper changes from 0 to +2, is this a redox reaction?
-No; copper gained 2 electrons and was reduced.
-Yes; copper lost 2 electrons and was oxidized.
-Yes, the products have 2 fewer electrons than the reactants.
-No, the products have as many electrons as the reactants.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
Study the reaction.
2AgNO3 (aq)+Cu (s)→Cu(NO3)2 (aq)+2Ag (s)
If the oxidation number of coppe...
If the oxidation number of coppe...
Questions



Mathematics, 07.05.2021 23:50


History, 07.05.2021 23:50


Social Studies, 07.05.2021 23:50


Mathematics, 07.05.2021 23:50

Mathematics, 07.05.2021 23:50



Mathematics, 07.05.2021 23:50

Mathematics, 07.05.2021 23:50


Mathematics, 07.05.2021 23:50

Mathematics, 07.05.2021 23:50

Physics, 07.05.2021 23:50


Mathematics, 07.05.2021 23:50