The information below describes a redox reaction.
Ag+ (aq) + Al(s) — Ag(s) + A13+ (aq)
Ag+ (a...

Chemistry, 20.05.2021 22:40 lilpeepxliltracy
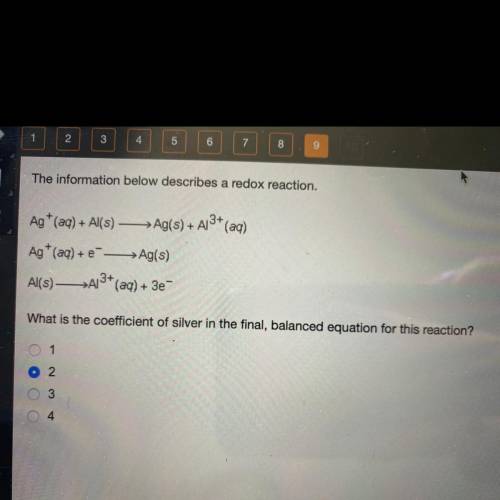
The information below describes a redox reaction.
Ag+ (aq) + Al(s) — Ag(s) + A13+ (aq)
Ag+ (aq) + --> Ag(s)
Al(s)—>A13+ (aq) + 3e-
What is the coefficient of silver in the final, balanced equation for this reaction?
1
2
3
4

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

You know the right answer?
Questions




Computers and Technology, 27.09.2019 23:30

English, 27.09.2019 23:30


Computers and Technology, 27.09.2019 23:30

Social Studies, 27.09.2019 23:30





Mathematics, 27.09.2019 23:30

Mathematics, 27.09.2019 23:30



English, 27.09.2019 23:30

Mathematics, 27.09.2019 23:30


Mathematics, 27.09.2019 23:30



