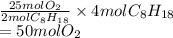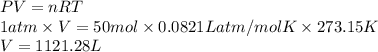Chemistry, 21.05.2021 16:30 kirstenb278
If 4.00 moles of gasoline are burned according to the chemical
reaction below, what volume of oxygen at STP is needed for complete
combustion?
2C2H18(I) + 25O2(g) → 16CO2(g) +18H2O(g)
Need done in 20 min if anyone could I help I’ll mark you as a brainiest!!

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
You know the right answer?
If 4.00 moles of gasoline are burned according to the chemical
reaction below, what volume of oxyge...
Questions


Geography, 14.12.2021 18:00

Mathematics, 14.12.2021 18:00





Mathematics, 14.12.2021 18:00



Mathematics, 14.12.2021 18:00


English, 14.12.2021 18:00





English, 14.12.2021 18:00


Chemistry, 14.12.2021 18:00

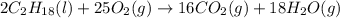
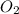 . Hence, moles of oxygen required to react with 4 moles of gasoline are calculated as follows.
. Hence, moles of oxygen required to react with 4 moles of gasoline are calculated as follows.