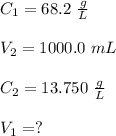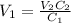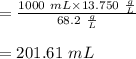Chemistry, 21.05.2021 17:50 aderahd7352
A stock solution has a concentration of 68.2 g/L. A 13.750 g/L solution is required. If you use a 1000.0 mL volumetric flask for the dilution, what volume (in ml) needs to be taken from the stock solution? Give your answer to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

You know the right answer?
A stock solution has a concentration of 68.2 g/L. A 13.750 g/L solution is required. If you use a 10...
Questions

Mathematics, 09.07.2019 10:10




Mathematics, 09.07.2019 10:10

Health, 09.07.2019 10:10





Mathematics, 09.07.2019 10:10

English, 09.07.2019 10:10

Mathematics, 09.07.2019 10:10


Business, 09.07.2019 10:10

Mathematics, 09.07.2019 10:10


Mathematics, 09.07.2019 10:10


Mathematics, 09.07.2019 10:10