
Chemistry, 21.05.2021 22:30 joelpimentel
A spirit burner used 1.00 g methanol to raise the temperature of 100.0 g water in a metal can from 28.00C to 58.0C. Calculate the heat of combustion in kJ/mol.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2

Chemistry, 23.06.2019 05:30
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1

Chemistry, 23.06.2019 07:00
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3
You know the right answer?
A spirit burner used 1.00 g methanol to raise the temperature of 100.0 g water in a metal can from 2...
Questions

Spanish, 05.05.2021 19:30





History, 05.05.2021 19:30

History, 05.05.2021 19:30





History, 05.05.2021 19:30


English, 05.05.2021 19:30



English, 05.05.2021 19:30

History, 05.05.2021 19:30


Mathematics, 05.05.2021 19:30

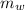 = 100 g
= 100 g


