

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

You know the right answer?
Propane is used as a fuel for camp stoves. It undergoes combustion to form carbon dioxide and water....
Questions


Mathematics, 10.09.2021 04:30

Biology, 10.09.2021 04:30



Mathematics, 10.09.2021 04:30

Mathematics, 10.09.2021 04:30


Chemistry, 10.09.2021 04:30

Mathematics, 10.09.2021 04:30




Chemistry, 10.09.2021 04:30


Chemistry, 10.09.2021 04:40

Mathematics, 10.09.2021 04:40


Mathematics, 10.09.2021 04:40

Mathematics, 10.09.2021 04:40


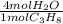 = 29.312 mol H₂O
= 29.312 mol H₂O

