
BONUS QUESTION
you are stuck with a problem. You need to measure pH of a solution known to be made from a metal hydroxide, but you don't have a meter or any indicators. You do happen to have some lead(II) nitrate that is soluble, and you remember that lead (II) hydroxide is insoluble. You add some to 1 liter of your own unkown solution and a precipitate forms. You add more unttil the precipitate stops forming and then a bit more just in case. After you filter and dry the precipitate, you have 3.81 grams of it. What was the approximate pH of the original solution?
*This is just a bonus question but I'm very confused on it so any help would be appreciated*

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

You know the right answer?
BONUS QUESTION
you are stuck with a problem. You need to measure pH of a solution known to be made...
Questions

English, 29.04.2022 09:30



Health, 29.04.2022 09:40



Mathematics, 29.04.2022 14:00






History, 29.04.2022 14:00

Mathematics, 29.04.2022 14:00

SAT, 29.04.2022 14:00

English, 29.04.2022 14:00




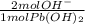 = 0.0316 mol OH⁻
= 0.0316 mol OH⁻


