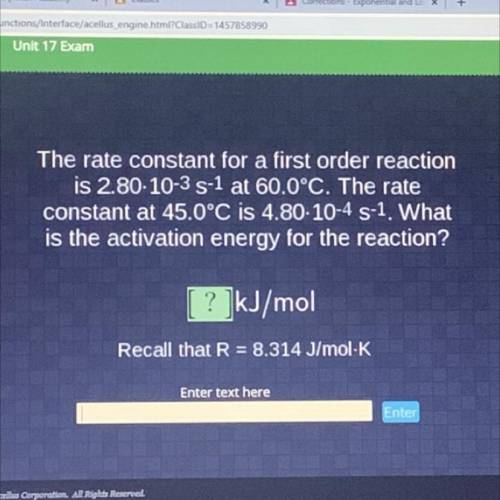
The rate constant for a first order reaction
is 2.80-10-35-1 at 60.0°C. The rate
constant at...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1


Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
Questions




History, 25.06.2019 06:30

History, 25.06.2019 06:30

Health, 25.06.2019 06:30








Mathematics, 25.06.2019 06:30


Biology, 25.06.2019 06:30


Physics, 25.06.2019 06:30