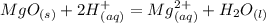Chemistry, 22.05.2021 18:10 mistycascaden
What is the ionic equation for this reaction:
MgO (s) + 2HCl (aq) = MgCl2 (aq) + H2O (l)
Please let me know how you worked it out, thankyou!!

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2
You know the right answer?
What is the ionic equation for this reaction:
MgO (s) + 2HCl (aq) = MgCl2 (aq) + H2O (l)
Questions




Social Studies, 06.06.2020 02:00

Mathematics, 06.06.2020 02:00


History, 06.06.2020 02:00

History, 06.06.2020 02:00

Physics, 06.06.2020 02:00





Mathematics, 06.06.2020 02:00

English, 06.06.2020 02:01