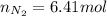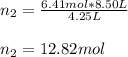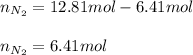Un recipiente cerrado, de 4,25 L, con tapa móvil, contiene H2S(g) a 740 Torr y 50,0°C. Se introduce en ese recipiente N2(g) a temperatura y presión constantes, de manera que el volumen final es el doble del volumen inicial. Calcular la cantidad de N2(g) en el recipiente, expresada en moles. porfi ayuda

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
You know the right answer?
Un recipiente cerrado, de 4,25 L, con tapa móvil, contiene H2S(g) a 740 Torr y 50,0°C. Se introduce...
Questions

Mathematics, 06.10.2020 14:01


Mathematics, 06.10.2020 14:01


Chemistry, 06.10.2020 14:01

Mathematics, 06.10.2020 14:01

Biology, 06.10.2020 14:01


Computers and Technology, 06.10.2020 14:01




Mathematics, 06.10.2020 14:01


Mathematics, 06.10.2020 14:01


Social Studies, 06.10.2020 14:01


Mathematics, 06.10.2020 14:01