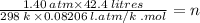Chemistry, 23.05.2021 14:00 ian2006huang
A sample of neon gas at 25.0°C has a volume of 42.4 L and a pressure of 1.40 atm. How many moles of neon are there? NOTE: You must show your calculation on the attached scratch paper,
including which of the Gas Law formulas you used. *
A. 14.4 moles
B. 0.41 moles
C. 28.9 moles
D. 2.43 moles
(Show how you did it please)

Answers: 3


Another question on Chemistry



Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 1
You know the right answer?
A sample of neon gas at 25.0°C has a volume of 42.4 L and a pressure of 1.40 atm. How many moles of...
Questions



Mathematics, 17.06.2020 20:57

Mathematics, 17.06.2020 20:57





English, 17.06.2020 20:57

Mathematics, 17.06.2020 20:57


Mathematics, 17.06.2020 20:57





Mathematics, 17.06.2020 20:57