
Chemistry, 23.05.2021 14:00 zahriaarana
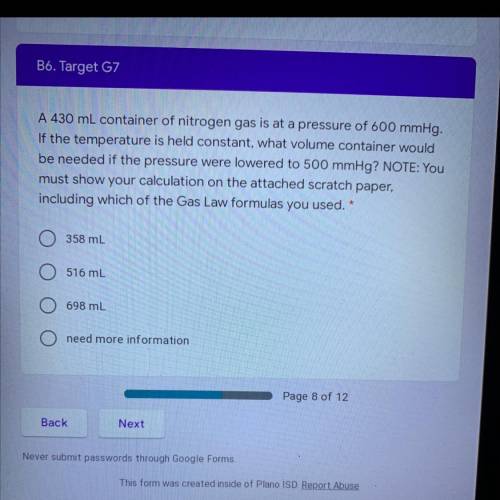
A 430 mL container of nitrogen gas is at a pressure of 600 mmHg.
If the temperature is held constant, what volume container would
be needed if the pressure were lowered to 500 mmHg? NOTE: You
must show your calculation on the attached scratch paper, including which of the Gas Law formulas you used.
A. 358 mL
B. 516 mL
C. 698 mL
D. need more information
(Show your work please)

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 04:10
In an experiment, 45g of silicon tetrachloride are treated with 45ml of water. what is the theoretical yield in grams of hcl
Answers: 3

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2

Chemistry, 23.06.2019 06:50
The student repeated the experiment using a higher concentration of acid. the same volume of acid and the same mass of magnesium ribbon were used. what volume of hydrogen gas would have been produced after 60 seconds?
Answers: 1
You know the right answer?
A 430 mL container of nitrogen gas is at a pressure of 600 mmHg.
If the temperature is held constan...
Questions



Mathematics, 06.04.2021 16:20

Biology, 06.04.2021 16:20




Mathematics, 06.04.2021 16:20



English, 06.04.2021 16:20


English, 06.04.2021 16:20


Mathematics, 06.04.2021 16:20





Social Studies, 06.04.2021 16:20



