
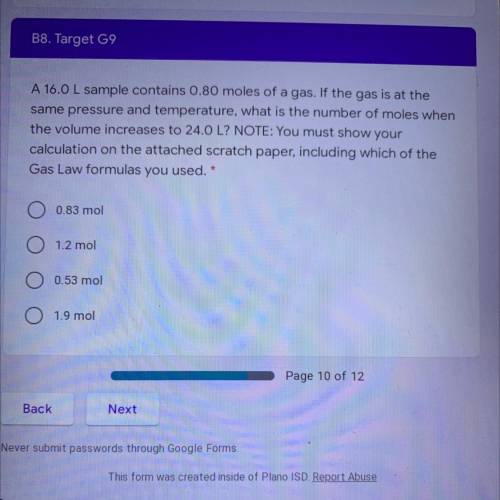
A 16.0 L sample contains 0.80 moles of a gas. If the gas is at the
same pressure and temperature, what is the number of moles when
the volume increases to 24.0 L? NOTE: You must show your
calculation on the attached scratch paper, including which of the
Gas Law formulas you used. *
A. 0.83 mol
B. 1.2 mol
C. 0.53 mol
D. 1.9 mol
(Please show your work)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

You know the right answer?
A 16.0 L sample contains 0.80 moles of a gas. If the gas is at the
same pressure and temperature, w...
Questions

Mathematics, 14.12.2019 02:31






Computers and Technology, 14.12.2019 02:31

Mathematics, 14.12.2019 02:31


Mathematics, 14.12.2019 02:31



Physics, 14.12.2019 02:31

Social Studies, 14.12.2019 02:31



English, 14.12.2019 02:31

Mathematics, 14.12.2019 02:31


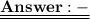
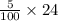 = 120/100= 12/10= 1.2 Mole ( Ans )
= 120/100= 12/10= 1.2 Mole ( Ans )

