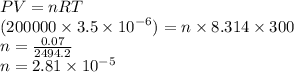Chemistry, 23.05.2021 21:00 thedarcieisabelleand
What is the number of moles in a sample of gas that has a volume of 3.5 L, a pressure of 200 kPa, and a temperature of 300 K?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2
You know the right answer?
What is the number of moles in a sample of gas that has a volume of 3.5 L, a
pressure of 200 kPa, a...
Questions

History, 02.11.2020 23:50

Mathematics, 02.11.2020 23:50



Biology, 02.11.2020 23:50

Mathematics, 02.11.2020 23:50

Mathematics, 02.11.2020 23:50

English, 02.11.2020 23:50




Mathematics, 03.11.2020 01:00

Health, 03.11.2020 01:00

Social Studies, 03.11.2020 01:00

Mathematics, 03.11.2020 01:00

Mathematics, 03.11.2020 01:00

Mathematics, 03.11.2020 01:00



Physics, 03.11.2020 01:00