
Chemistry, 23.05.2021 22:30 jeancarlo1107
In PbO + 2HCl → PbCl2 + H20, 13 g of lead oxide is
mixed with 6.4 g of hydrochloric acid to produce lead chloride
and water. Which of the following is true?
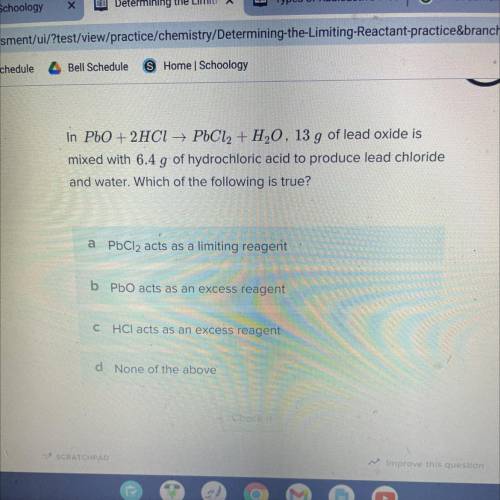

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1


Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1

Chemistry, 23.06.2019 10:30
How much mass would a mole of hydrogen molecules contain? recall that hydrogen is diatomic. g/mol
Answers: 3
You know the right answer?
In PbO + 2HCl → PbCl2 + H20, 13 g of lead oxide is
mixed with 6.4 g of hydrochloric acid to produce...
Questions

Mathematics, 03.09.2020 03:01


Chemistry, 03.09.2020 03:01




History, 03.09.2020 03:01

Computers and Technology, 03.09.2020 03:01

Biology, 03.09.2020 03:01

Mathematics, 03.09.2020 03:01


Mathematics, 03.09.2020 03:01


English, 03.09.2020 03:01

Biology, 03.09.2020 03:01



Biology, 03.09.2020 03:01

Business, 03.09.2020 03:01

Biology, 03.09.2020 03:01



