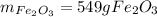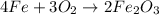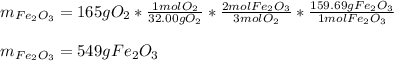Chemistry, 23.05.2021 23:30 elizabethxoxo3271
How many grams of iron oxide, Fe2O3 will be produced if 165 g of O2 gas is supplied? (follow the same steps as mol to mol, only now your flow should be like this: grams O2 moles O2 moles Fe2O3 grams Fe2O3 Fe + O2 Fe2O3

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
A__ is two or more substances that are together in the same place but are not chemically combined
Answers: 3

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 24.06.2019 07:30
Whose work directly resulted in the development of an atomic model that has negative electrons stuck with a sea of positive material?
Answers: 3

Chemistry, 24.06.2019 09:00
What is an aspect of the kinetic molecular theory and can be used to explain the compressibility of plasmas?
Answers: 1
You know the right answer?
How many grams of iron oxide, Fe2O3 will be produced if 165 g of O2 gas is supplied? (follow the sam...
Questions

English, 29.01.2020 00:58

Mathematics, 29.01.2020 00:58

Social Studies, 29.01.2020 00:58



History, 29.01.2020 00:58

History, 29.01.2020 00:58

English, 29.01.2020 00:58







Mathematics, 29.01.2020 00:58

Mathematics, 29.01.2020 00:58



Biology, 29.01.2020 00:58