
Chemistry, 24.05.2021 07:30 lovelife132015
You react hydrogen gas with chlorine gas according to the following reaction:
H2(g) * Cl2(g) -> 2HCl(g)
What mass of HCl(g) can be produced from 2.5 * 10^3 g of hydrogen gas with an excess of chlorine gas?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1
You know the right answer?
You react hydrogen gas with chlorine gas according to the following reaction:
H2(g) * Cl2(g) ->...
Questions



Mathematics, 13.12.2020 03:30


Mathematics, 13.12.2020 03:30

Mathematics, 13.12.2020 03:30

Chemistry, 13.12.2020 03:30


Biology, 13.12.2020 03:30

English, 13.12.2020 03:30

Mathematics, 13.12.2020 03:30


Mathematics, 13.12.2020 03:30

Mathematics, 13.12.2020 03:30


History, 13.12.2020 03:30



Arts, 13.12.2020 03:30

Mathematics, 13.12.2020 03:30

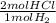 = 2500 mol HCl
= 2500 mol HCl

