
Chemistry, 24.05.2021 18:20 michaelchavez6959127
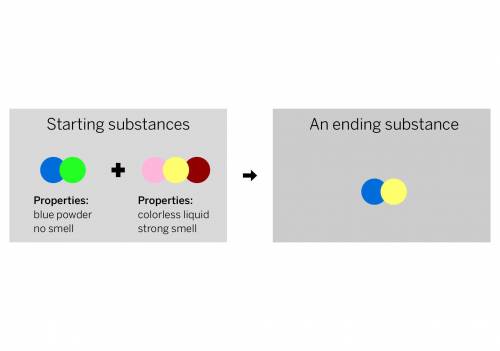
A chemist mixed two substances together: a blue powder with no smell and a colorless liquid with a strong smell. Their repeating groups of atoms are shown above on the left. After they were mixed, the chemist analyzed the results and found two substances. One ending substance had the repeating group of atoms shown above on the right. Is the ending substance the same substance as the blue powder? What happened to the atoms of the starting substances when the ending substances formed? Be sure to explain your answers to both of these questions.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3


You know the right answer?
A chemist mixed two substances together: a blue powder with no smell and a colorless liquid with a s...
Questions





English, 24.06.2019 03:50

Biology, 24.06.2019 03:50


English, 24.06.2019 03:50



Biology, 24.06.2019 03:50


Mathematics, 24.06.2019 04:00



Business, 24.06.2019 04:00

Biology, 24.06.2019 04:00

Biology, 24.06.2019 04:00




