Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2
You know the right answer?
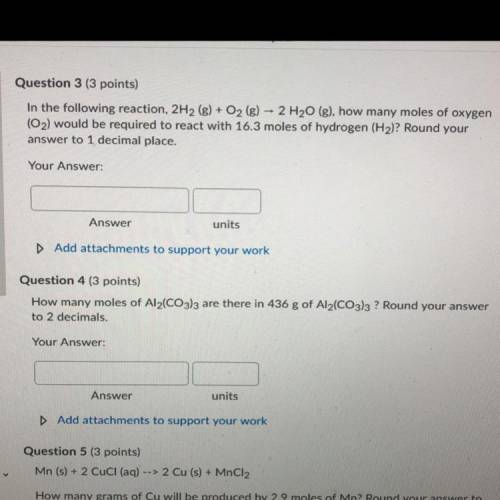
In the following reaction, 2H2 (g) + O2 (g) + 2 H2O (g), how many moles of oxygen
(O2) would be req...
Questions

Mathematics, 08.06.2021 17:50

Social Studies, 08.06.2021 17:50


Mathematics, 08.06.2021 17:50






Mathematics, 08.06.2021 17:50

Business, 08.06.2021 17:50

Mathematics, 08.06.2021 17:50

Chemistry, 08.06.2021 17:50

Mathematics, 08.06.2021 17:50


Mathematics, 08.06.2021 17:50

Mathematics, 08.06.2021 17:50

Mathematics, 08.06.2021 17:50