
Chemistry, 24.05.2021 21:40 jadejordan8888
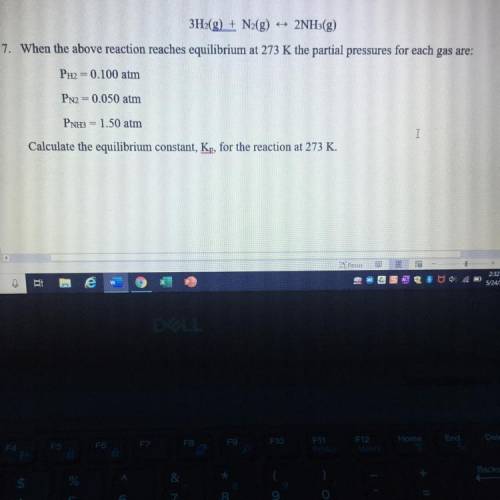
3H2(g) + N2(g) 2NH3(g)
When the above reaction reaches equilibrium at 273 K the partial pressures for each gas are:
PH2 = 0.100 atm
PN2 = 0.050 atm
PNH3 = 1.50 atm
Calculate the equilibrium constant, Kp, for the reaction at 273 K.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:50
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1


Chemistry, 23.06.2019 16:00
Which question comparing two different metals is best answered through science? a. which metal is easier to use? b. which metal is harder to find? c. which metal is less dense? d. which metal is more valuable?
Answers: 2

Chemistry, 23.06.2019 18:30
Match the following items. match the items in the left column to the items in the right column. 1. 1/1,000 precision 2. uncertainty value of measurement milli- 3. 1,000 accuracy 4. instrument to measure volume balance 5. degree of exactness of a measurement centi- 6. instrument to measure mass graduated cylinder 7. correctness of a measurement ± value 8. 1/100 kilo-
Answers: 1
You know the right answer?
3H2(g) + N2(g) 2NH3(g)
When the above reaction reaches equilibrium at 273 K the partial pressures f...
Questions

English, 21.04.2020 23:44

Mathematics, 21.04.2020 23:44



Arts, 21.04.2020 23:44


History, 21.04.2020 23:44


Mathematics, 21.04.2020 23:44

Engineering, 21.04.2020 23:44

Mathematics, 21.04.2020 23:44




Computers and Technology, 21.04.2020 23:44


Mathematics, 21.04.2020 23:44

Mathematics, 21.04.2020 23:44

Advanced Placement (AP), 21.04.2020 23:44




