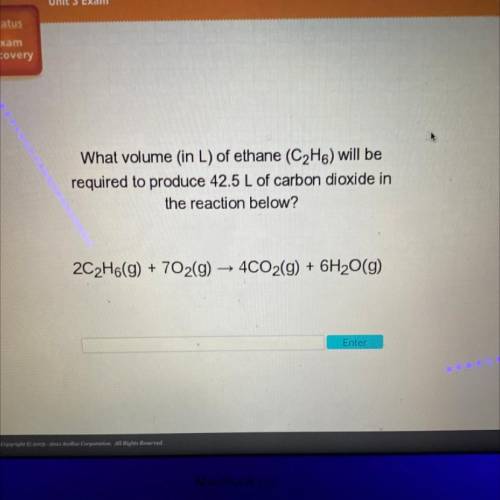
What volume (in L) of ethane (C2H6) will be
required to produce 42.5 L of carbon dioxide in
t...

Chemistry, 25.05.2021 01:00 FavvBella84
What volume (in L) of ethane (C2H6) will be
required to produce 42.5 L of carbon dioxide in
the reaction below?
2C2H6(g) + 702(g) → 4CO2(g) + 6H2O(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 23.06.2019 03:30
Ineed pls urgent 1-20 in order and fully detail step my step.
Answers: 1

Chemistry, 23.06.2019 08:00
Problem page a jet airplane reaches 846. km/h on a certain flight. what distance does it cover in 13.0 min? set the math up. but don't do any of it. just leave your answer as a math expression. also, be sure your answer includes all the correct unit symbols.
Answers: 2
You know the right answer?
Questions

English, 12.02.2022 20:50

Social Studies, 12.02.2022 20:50

English, 12.02.2022 20:50



Mathematics, 12.02.2022 20:50


Biology, 12.02.2022 20:50

Mathematics, 12.02.2022 20:50

English, 12.02.2022 20:50

English, 12.02.2022 20:50

English, 12.02.2022 20:50

Mathematics, 12.02.2022 20:50

Mathematics, 12.02.2022 20:50

English, 12.02.2022 20:50


Mathematics, 12.02.2022 20:50

History, 12.02.2022 20:50




