
Chemistry, 25.05.2021 01:00 NetherisIsTheQueen
In an aqueous chloride solution cobalt(II) exists in equilibrium with the complex ion CoCl42-. Co2 (aq) is pink and CoCl42-(aq) is blue. At Low Temperature the pink color pre-dominates. At High Temperature the blue color is strong. If we represent the equilibrium as:
CoCl4^2-(aq) <--> Co2+(aq) + 4Cl-(aq)
We can conclude that:___.
1. This reaction is:___.
A. Exothermic
B. Endothermic
C. Neutral
2. When the temperature is decreased the equilibrium constant, K:.
A. Increases
B. Decreases
C. Remains the same.
3. When the temperature is decreased the equilibrium concentration of Co2:.
A. Increases
B. Decreases
C. Remains the same.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 23.06.2019 06:30
Which of the following is true about the products formed during photosynthesis? (5 points) select one: a. they have the same mass as the mass of reactants. b. they are the same set of compounds as the reactants. c. they have more mass than the mass of reactants. d. they are chemically the same as the reactants.
Answers: 1
You know the right answer?
In an aqueous chloride solution cobalt(II) exists in equilibrium with the complex ion CoCl42-. Co2 (...
Questions


Mathematics, 29.09.2020 03:01


Chemistry, 29.09.2020 03:01


Mathematics, 29.09.2020 03:01


Social Studies, 29.09.2020 03:01


Mathematics, 29.09.2020 03:01


Mathematics, 29.09.2020 03:01


Arts, 29.09.2020 03:01

Engineering, 29.09.2020 03:01




Biology, 29.09.2020 03:01

History, 29.09.2020 03:01


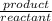 =
=![\frac{[CO^2^+][Cl^-^4]}{CoCl^2^-_4}](/tpl/images/1345/2628/18cbc.png)
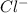 and
and  rises, and K rises as well , thus it increases .
rises, and K rises as well , thus it increases .

