
Chemistry, 25.05.2021 01:00 gayshipsandcoffee
Mole Conversion -
The researcher measures 6.5 moles of Magnesium Chloride (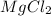 ). Using balanced equation Mg + 2 HCl --->
). Using balanced equation Mg + 2 HCl ---> 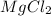 +
+ 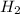 , how many moles of HCl were in the sample?
, how many moles of HCl were in the sample?
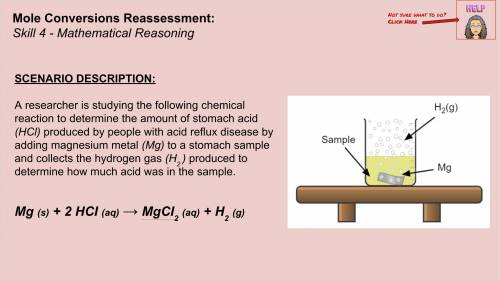

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
Mole Conversion -
The researcher measures 6.5 moles of Magnesium Chloride (). Using balanced equati...
Questions

Chemistry, 28.03.2021 20:50



English, 28.03.2021 20:50



Mathematics, 28.03.2021 20:50

Mathematics, 28.03.2021 20:50


Mathematics, 28.03.2021 20:50

Mathematics, 28.03.2021 20:50


Mathematics, 28.03.2021 20:50

Mathematics, 28.03.2021 20:50



Mathematics, 28.03.2021 20:50

Social Studies, 28.03.2021 21:00

Social Studies, 28.03.2021 21:00

Mathematics, 28.03.2021 21:00



