Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
You know the right answer?
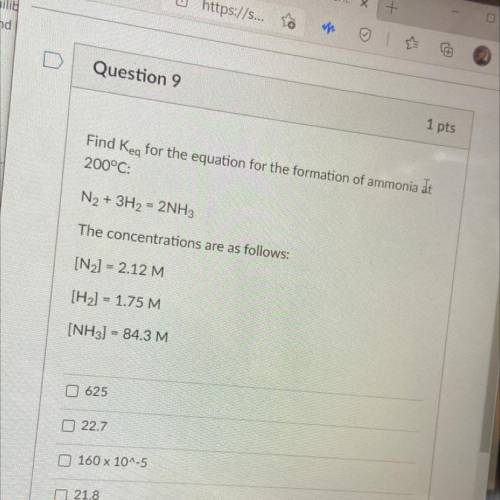
Find Key for the equation for the formation of ammonia at
200°C:
N2 + 3H2 = 2NH3
The co...
N2 + 3H2 = 2NH3
The co...
Questions

Geography, 29.01.2020 12:57



Biology, 29.01.2020 12:57

Computers and Technology, 29.01.2020 12:57


Social Studies, 29.01.2020 12:57


Mathematics, 29.01.2020 12:57


Advanced Placement (AP), 29.01.2020 12:57


Mathematics, 29.01.2020 12:57

English, 29.01.2020 12:57

Mathematics, 29.01.2020 12:57



Mathematics, 29.01.2020 12:57

Mathematics, 29.01.2020 12:57

Mathematics, 29.01.2020 12:57