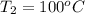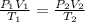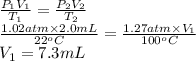Chemistry, 25.05.2021 18:10 Thisisdifinite
A 2.0 mL sample of air in a syringe exerts a pressure of 1.02 atm at 22 C. If that syringe is placed into boiling water at 100 C and the pressure in the syringe increases to 1.27 atm, what is the new volume of the air in the syringe?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
You know the right answer?
A 2.0 mL sample of air in a syringe exerts a pressure of 1.02 atm at 22 C. If that syringe is placed...
Questions

Mathematics, 23.08.2019 12:30



History, 23.08.2019 12:30




Chemistry, 23.08.2019 12:30


French, 23.08.2019 12:30

Social Studies, 23.08.2019 12:30



Biology, 23.08.2019 12:30

Mathematics, 23.08.2019 12:30

History, 23.08.2019 12:30




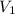 = 2.0 mL,
= 2.0 mL, 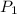 = 1.02 atm,
= 1.02 atm, 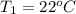
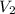 = ?,
= ?, 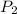 = 1.27 atm,
= 1.27 atm,