
Chemistry, 25.05.2021 18:40 OkayLearn5522
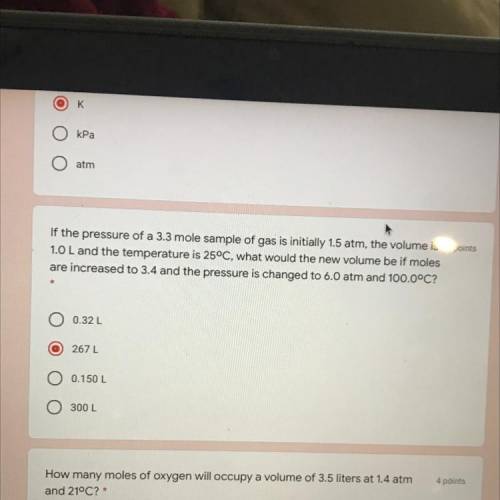
If the pressure of a 3.3 mole sample of gas is initially 1.5 atm, the volume is 4 points
1.0 L and the temperature is 25°C, what would the new volume be if moles
are increased to 3.4 and the pressure is changed to 6.0 atm and 100.0°C?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
If the pressure of a 3.3 mole sample of gas is initially 1.5 atm, the volume is 4 points
1.0 L and...
Questions


Mathematics, 12.03.2020 09:50



Mathematics, 12.03.2020 09:53

Mathematics, 12.03.2020 09:53

Physics, 12.03.2020 09:53


Mathematics, 12.03.2020 09:54








Mathematics, 12.03.2020 09:56

Mathematics, 12.03.2020 09:57





