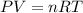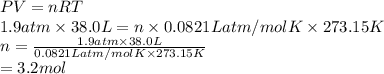Chemistry, 25.05.2021 19:00 beanokelley
How many moles of gas would you have if you have a volume of 38.0 L under a pressure of 1430 mmHg at standard temperature

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1
You know the right answer?
How many moles of gas would you have if you have a volume of 38.0 L under a pressure of 1430 mmHg at...
Questions

Geography, 07.04.2020 06:35




Biology, 07.04.2020 06:35



Mathematics, 07.04.2020 06:36

Mathematics, 07.04.2020 06:36


Mathematics, 07.04.2020 06:38

Mathematics, 07.04.2020 06:38


Mathematics, 07.04.2020 06:39



Mathematics, 07.04.2020 06:40

Biology, 07.04.2020 06:40

History, 07.04.2020 06:40

Mathematics, 07.04.2020 06:40