Given the reaction:
4NH3(g) + 502(g) → 4NO(g) + 6H2O(g)
At constant pressure, how many liters...

Chemistry, 25.05.2021 19:20 issacbeecherpebpyl
Given the reaction:
4NH3(g) + 502(g) → 4NO(g) + 6H2O(g)
At constant pressure, how many liters of O2 (g)
would be required to produce 20 liters of NO (g)?
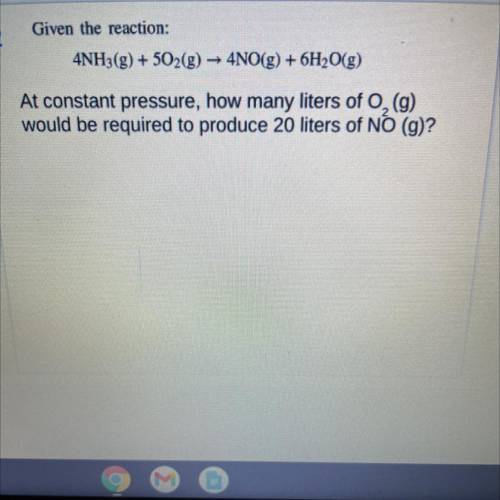

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2
You know the right answer?
Questions



Mathematics, 04.12.2020 23:10


History, 04.12.2020 23:10


Mathematics, 04.12.2020 23:10



Social Studies, 04.12.2020 23:10





Physics, 04.12.2020 23:10

Mathematics, 04.12.2020 23:10

Mathematics, 04.12.2020 23:10


Mathematics, 04.12.2020 23:10

Computers and Technology, 04.12.2020 23:10



