Chemistry, 25.05.2021 22:40 AbhiramAkella
How many joules of heat are needed to raise the temperature of 30.0 g of aluminum from 22°C to 80°C, if the specific heat of aluminum is 0.90 J/g°C?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:30
One of the reactions in a blast furnace used to reduce iron is shown above. how many grams of fe2o3 are required to produce 15.5 g of fe if the reaction occurs in the presence of excess co? a.11.1 g b.22.1 g c.30.0 g d.44.2 g
Answers: 2

Chemistry, 21.06.2019 16:30
What is a scientific theory? a. a scientist's guess about how something works b. the results of an experiment obtained using the scientific method c. a proven fact that will never change d. an idea that is backed by data from many sources
Answers: 2

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1
You know the right answer?
How many joules of heat are needed to raise the temperature of 30.0 g of aluminum from 22°C to 80°C,...
Questions

Geography, 22.04.2021 02:30

Mathematics, 22.04.2021 02:30



Spanish, 22.04.2021 02:30

Mathematics, 22.04.2021 02:30

English, 22.04.2021 02:30





History, 22.04.2021 02:30

Mathematics, 22.04.2021 02:30

Mathematics, 22.04.2021 02:30


Mathematics, 22.04.2021 02:30

Mathematics, 22.04.2021 02:30

Mathematics, 22.04.2021 02:30


Physics, 22.04.2021 02:30

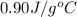
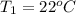
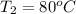
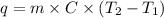
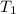 = initial temperature
= initial temperature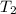 = final temperature
= final temperature